2020-11-21 by Nick Connor Atomic Mass of Carbon Atomic mass of Carbon is 12.0107 u. This is a list of the 118 chemical elements which have been identified as of 2021. A chemical element, often simply called an element, is a species of atoms which all have the same number of protons in their atomic nuclei (i.e., the same atomic number, or Z). Atomic no of carbon is 6 Because the mass of the atom is made up of the number of protons and neutrons, carbon also has six neutrons in its nucleus. It has six electrons surrounding the nucleus, with two in the first energy level and four in the second energy level. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, 12 C, that is taken as a standard element with an atomic mass of 12. This isotope of carbon has 6 protons and 6 neutrons. Thus, each proton and neutron has a mass of about 1 amu.
CARBON FIBER INFUSED Deep Black Opaque Extreme PETG PRO
Available in ONE KILO (2.2lbs) & 3.5 KG Jumbo rolls
Prints with a deep black slightly textured surface & no transparency.
Filament and parts are NOT brittle like some other CF products. Excellent for professional looking technical prints.
Engineering grade PETG resin with USA made premium MILLED carbon fiber NOT powder or dust.
PETG is a newer printing filament and has the advantage of printing easily like PLA, but offering higher temp and impact resistance like ABS.
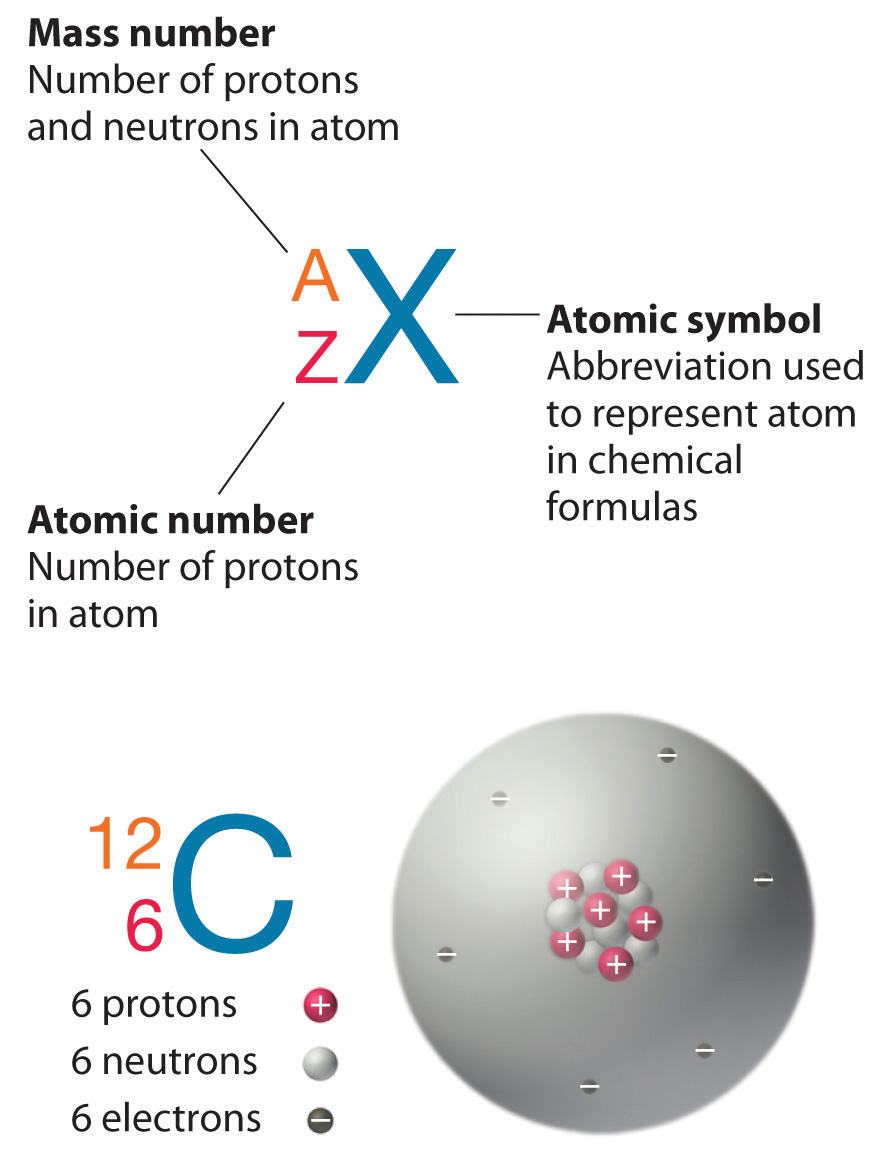
Carbon fiber infused filament benefits:
- Increased rigidity / strength
- Highly desirable texture / look
- Greatly reduced part shrink / warp
- Greatly reduced stringing / angel hairs
- Printed parts hold better details / sharp corners
Quality / Specs
Print nozzle temp - 240 - 265C
Print nozzle size - 0.40mm or larger
Heated Bed temp - 60 - 78c
Diameter (volumetric variation XY combined) + - 0.02mm or better
Roundness ( ovality ) + - 0.02 mm or better
* mild abrasive to print nozzles.
How do you calculate the atomic mass of carbon?
/human-body-composition-59021b3c5f9b5810dc784933.jpg)
1 Answer
The term 'atomic mass' refers to the mass of a single atom. The mass of a single atom of carbon-12 is defined as exactly 12 u.
The term atomic mass is also often used (though technically, incorrectly) to refer to the average atomic mass of all of the isotopes of an element.
This second definition is actually the relative atomic mass of an element — a single average value of the element's mass based on the masses of its isotopes.
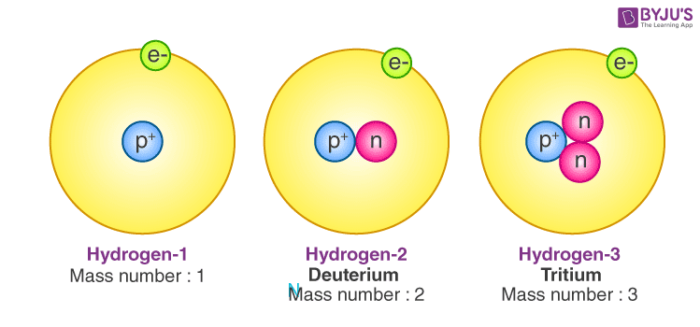
Carbon has 15 known isotopes, of which only two (
Carbon consists of 98.93%
METHOD 1
To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms.
Assume that you have, say, 10 000 atoms of carbon. Then you have 9893 atoms of
Cached
METHOD 2
Another way of determining the average mass is to multiply the atomic mass of each isotope by its percentage and then add the numbers.
The two methods are mathematically equivalent. Thus,
Method 2 is probably mathematically simpler, but Method 1 makes it clear that you are determining an average mass.
Choose the method that you prefer.
What Is A Carbon Atom
Related questions
